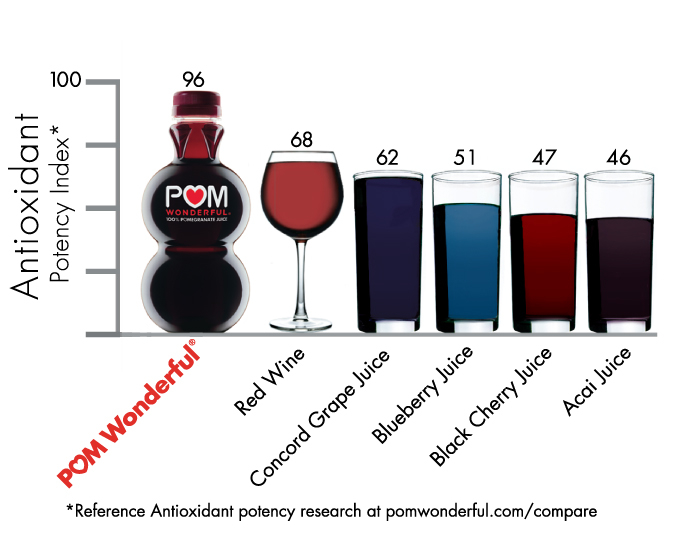
 The, self-proclaimed, healthy beverage POM is now involved in a suit by the FTC. The beverage has been accused of misleading consumers with its ads making specific health claims.
The, self-proclaimed, healthy beverage POM is now involved in a suit by the FTC. The beverage has been accused of misleading consumers with its ads making specific health claims.Some of the ads claimed the beverage could help decrease arterial plaque by 30 percent and improve blood flow by 17 percent. It is noted in the "Marketers Wary Following FTC/POM Dispute" article that both the FTC and the FDA felt as though they gave POM ample opportunity to changer their ads or provide the research to support them. The beverage company is now required to cease in their advertising and have any further advertising material approved by the agencies before releasing to the public.
POM was warned and continued their marketing campaign. I think the company should head a warning next time instead of continuing. Contrary to many beliefs, not all publicity is good publicity.
No comments:
Post a Comment